An anticoagulant peptide from beta-casein: identification, structure and molecular mechanism
Abstract
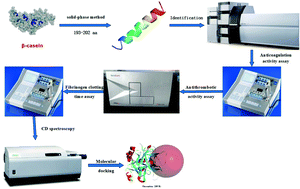
Various bioactive peptides are identified from casein hydrolysates. YQEPVLGPVR (PICA), a novel antithrombotic peptide derived from beta-casein (fragment 193–202), was identified by high-performance liquid chromatography – liquid chromatography-mass spectrometry/mass spectrometry. The anticoagulation activity assay showed that this peptide has a strong anticoagulant activity. It was proved that the peptide did not interact with the active site of thrombin to inhibit thrombin, and that it inhibited thrombin activity by binding the exosite-1 of thrombin, which was also confirmed by the fibrinogen clotting time assay. It was shown that PICA prolonged fibrinogen clotting time in a dose-dependent manner. Secondary structures of the thrombin–PICA complex were also measured by circular dichroism to prove that PICA can combine with thrombin. Moreover, Discovery Studio 2017 R2 software was used for molecular docking to provide the potential mechanism for the antithrombotic activity of the peptide. These results suggested that PICA probably can be used as an antithrombotic ingredient in the functional food industry.


 Please wait while we load your content...
Please wait while we load your content...