Impact of enzymatic hydrolysis followed by transglutaminase-induced cross-linking on decreasing antigenicity and reserving partial interfacial properties of whey protein isolate
Abstract
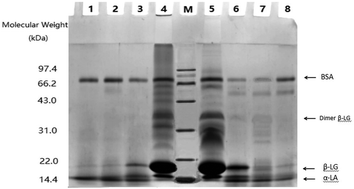
Whey protein isolate (WPI) was hydrolyzed by alcalase and trypsin for three hydrolysis degrees (DHs), followed by transglutaminase (TGase) induced cross-linking. The prepared products were measured for surface hydrophobicity and emulsifying and foaming properties, as well as in vitro antigenicity for α-lactalbumin and β-lactoglobulin. The results indicated that enzymatic hydrolysis of WPI mostly resulted in WPI hydrolysates with significantly decreased antigenicity of α-lactalbumin and β-lactoglobulin, especially in the case of a higher DH value. Moreover, the TGase-induced cross-linking led to a further antigenicity decrease for these prepared products. Alcalase was always more potent than trypsin to decrease antigenicity. In comparison with WPI, the conducted enzymatic hydrolysis also brought losses to surface hydrophobicity and emulsifying and foaming properties. On the other hand, the conducted cross-linking could partially rescue these properties. It is thus concluded that the assessed enzymatic hydrolysis coupled with TGase-induced cross-linking might be an applicable process for WPI to decrease its potential antigenicity but reserve partial interfacial properties.


 Please wait while we load your content...
Please wait while we load your content...