β-Secretase (BACE1) inhibitory and neuroprotective effects of p-terphenyls from Polyozellus multiplex
Abstract
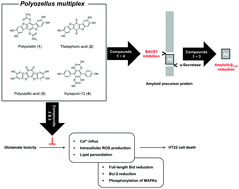
Alzheimer's disease (AD), a major neurodegenerative disorder, is associated with the enzymatic reaction of β-secretase (BACE1) on the amyloid precursor protein (APP) for the generation of neurotoxic amyloid-β (Aβ). Therefore, Aβ accumulation and oxidative stress-induced neuronal cell death are the pathogenic hallmarks of AD. In this study, we tried to identify BACE1 inhibitors and neuroprotectants from natural products, in particular, from the Korean mushroom Polyozellus multiplex. Four p-terphenyls were identified from the ethanolic extract of P. multiplex; polyozellin (1), thelephoric acid (2), polyozellic acid (3), and kynapcin-12 (4). Compounds 1–4 effectively inhibited BACE1 activity with a half-maximal inhibitory concentration (IC50) of 3.08, 3.50, 4.78, and 15.79 μM, respectively. Compounds 1–3 reduced the production of neurotoxic Aβ1-42 production in APPswe-N2a cells in a concentration-dependent manner. When HT22 cells were stressed with 5 mM glutamate, compounds 2 and 3 significantly recovered cell viability. It was correlated with their inhibitory properties against glutamate-mediated Ca2+ influx, intracellular reactive oxygen species (ROS) generation, lipid peroxidation, reduction in Bcl-2 and Bid levels, and enhanced phosphorylation of mitogen-activated protein kinase (MAPK). Thus, P. multiplex and the isolated p-terphenyls might be useful in the development of lead compounds for the prevention of neurodegenerative diseases, especially AD.

 Please wait while we load your content...
Please wait while we load your content...