Solubilization by freeze-milling of water-insoluble subunits in rice proteins
Abstract
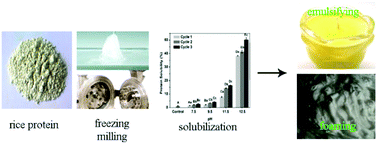
This study investigates the effects of freeze-milling on the structural and functional properties of rice proteins (RPs). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that freeze-milling slightly influences the subunit bands of the RPs. Secondary and tertiary structures were studied by analyzing Fourier transform infrared spectra, sulfhydryl and disulfide bond contents, and surface hydrophobicities. The freeze-milled RPs (FMRPs) may possess an unfolded conformation that exposes buried functional groups. In addition, the solubility of the FMRPs is higher than that of the control, probably due to the exposure of water–protein interaction areas. In particular, the solubility of the FMRPs treated at a pH of 12.5 was 42 times that of the control. Characterization of functionalities demonstrated that both the emulsifying and foaming activities of the FMRPs were improved by solubilization. However, functional stabilities either remained unaffected or deteriorated. Generally, the FMRPs showed better emulsifying activities and stabilities than bovine serum albumin, alongside better foaming activities and stabilities than hen egg albumin. FMRPs may be of great interest to the food industry.

 Please wait while we load your content...
Please wait while we load your content...