Evaluation of the biointeraction of colorant flavazin with human serum albumin: insights from multiple spectroscopic studies, in silico docking and molecular dynamics simulation†
Abstract
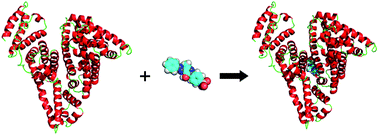
Azo compounds are the largest chemical class of agents frequently used as colorants in a variety of consumer goods and farm produce; therefore, they may become a hazard to public health, because numerous azo compounds and their metabolites are proven to be carcinogens and mutagens. Herein several qualitative and quantitative analytical techniques, including steady state and time-resolved fluorescence, circular dichroism (CD), computer-aided molecular docking as well as molecular dynamics simulation, were employed to ascertain the molecular recognition between the principal vehicle of ligands in human plasma, albumin and a model azo compound, flavazin. The results show that the albumin spatial structure was changed in the presence of flavazin with a decrease of α-helix suggesting partial protein destabilization/self-regulation, as derived from steady state fluorescence, far-UV CD and detailed analyses of three-dimensional fluorescence spectra. Time-resolved fluorescence further evinced that the recognition mechanism is related to albumin–flavazin adduct formation with an association intensity of 104 M−1, and the driving forces were found to be chiefly π–π interactions, hydrophobic interactions and hydrogen bonds. The specific binding domain of flavazin in protein was defined from molecular docking; subdomain IIA (Sudlow's site I) was found to retain high affinity for the ligand flavazin. This finding corroborates the results of competitive ligand displacement experiments, a hydrophobic 8-anilino-1-naphthalenesulfonic acid probe study and protein denaturation results, placing flavazin at the warfarin–azapropazone site. Based on molecular dynamics simulation, it can be said with certainty that the results of molecular docking are credible, and the key amino acid residues participating in the molecular recognition of flavazin by protein are clearly Trp-214, Arg-222 and Lys-436. The outcomes presented here will help to further comprehend the molecular recognition of azo compounds by protein and the possible toxicological profiles of other compounds that have configurations analogous to azo chemicals.

 Please wait while we load your content...
Please wait while we load your content...