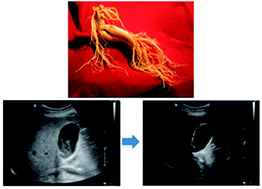
Although ginseng, the root of Panax quinquefolium and P. ginseng, was reported to have anti-cholelithogenic effects in animal experiments, there have, to date, been no human studies. We conducted this prospective, controlled, double-blind pilot trial to evaluate the safety and efficiency of Korean red ginseng (KRG), the steamed root of P. ginseng C.A. Meyer. Twenty eight consecutive patients were randomized to receive either KRG (7.5 g divided into three daily doses) or a placebo as an adjuvant to the standard regimen of bile acids for gallstones (500 mg of chenodeoxycholic acid and 500 mg of ursodeoxycholic acid divided into three daily doses) for 24 weeks. No case of serious adverse reaction occurred in both groups. Although the decrease in stone burden was larger in the KRG group (3.4 ± 0.6 ml3) than in the placebo group (2.3 ± 1.1 ml3), it did not reach statistical significance (p = 0.09). Also there were no differences in the rate of complete dissolution, subjective improvement in symptoms, and the rate of cholecystectomy due to worsening pain or the development of complications and changes in laboratory tests before and after treatment. In conclusion, the addition of KRG as an adjuvant was safe for patients undergoing bile acid dissolution therapy for gallstones although it did not affect the results. Large-scaled trials to optimize regimens are expectantly needed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...