Sol–gel synthesis of 2-dimensional TiO2: self-assembly of Ti–oxoalkoxy–acetate complexes by carboxylate ligand directed condensation†
Abstract
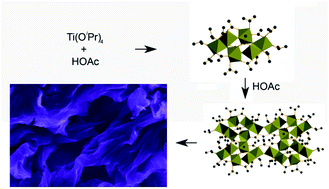
2-Dimensional (2D) metal oxides have many potential industrial applications including heterogeneous catalysis, water splitting, renewable energy conversion, supercapacitor applications, biomaterials, gas separation and gas storage. Herein we report a simple and scalable method for the preparation of 2D TiO2 nanostructures by reaction of titanium isopropoxide with acetic acid at 333 K in isopropanol, followed by calcination at 673 K to remove the organic ligands. Both the products and reaction intermediates have been studied using electron microscopy, X-ray diffraction, N2 physisorption, nuclear magnetic resonance, thermogravimetric analysis, and X-ray photoelectron, Raman, and infrared spectroscopy. The anisotropic condensation of the planar Ti6O4(OiPr)8(OAc)8 complex is believed to be responsible for the formation of the 2D structure, where OiPr and OAc represent isopropoxide and acetate ligands, respectively. This research demonstrates that the metal complexes are promising building blocks for desired architectures, and the self-assembly of an acetate bidentate ligand is a versatile tool for manipulating the shape of final products.
- This article is part of the themed collection: Chemistry of 2-dimensional materials: beyond graphene


 Please wait while we load your content...
Please wait while we load your content...