The influence of hydrofluoric acid etching processes on the photocatalytic hydrogen evolution reaction using mesoporous silicon nanoparticles†
Abstract
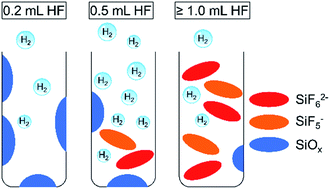
H2 has been identified as one of the potential energy vectors that can provide a sustainable energy supply when produced through solar-driven water-splitting reaction. Si is the second most abundant element in the Earth’s crust and can absorb a significant fraction of the solar spectrum while presenting little toxicity risk, making it an attractive material for photocatalytic H2 production. Hydrogen-terminated mesoporous Si (mp-Si) nanoparticles can be utilized to effectively drive the hydrogen evolution reaction using UV-to-visible light. In this work, the response of the photocatalytic activity of mp-Si nanoparticles to a series of HF acid treatments was investigated. A two-step magnesiothermic reduction method was used to prepare crystalline mp-Si nanoparticles with a specific surface area of 573 m2 g−1. The HF etching process was optimized as a function of the amount of acid added and the reaction time. The reaction time did not influence the H2 evolution rate substantially. However, the amount of HF used did have a significant effect on the photocatalytic activity. In the presence of ≥1.0 mL HF acid per 0.010 g of Si, morphological damage was observed using electron microscopy. N2 adsorption measurements indicated that the pore size and surface area were also altered. Solution-phase 19F{1H} NMR studies indicated the formation of SiF5− and SiF62− when larger volumes of HF were used. Both factors, morphological damage and the presence of byproducts in the pores, likely result in a lowering of the photocatalytic H2 evolution rate. Under the optimized HF treatment conditions (0.5 mL of HF per 0.010 g of Si), a H2 evolution rate of 1398 ± 30 μmol g−1 h−1 was observed.
- This article is part of the themed collection: Luminescent silicon nanostructures


 Please wait while we load your content...
Please wait while we load your content...