Elucidating cation effects in homogeneously catalyzed formic acid dehydrogenation†
Abstract
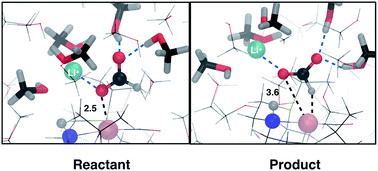
In this work, we use density functional theory based molecular dynamics with an explicit description of methanol solvent to study the effect of cations on formic acid dehydrogenation catalyzed by a ruthenium PNP pincer complex (RuPNP). Formic acid dehydrogenation is a two step process that involves the reorientation of the formate moiety bound via its oxygen to a H bound intermediate, followed by the hydride transfer step to form CO2 and the hydrogenated catalyst. We find the reorientation step to proceed with a low barrier in methanol solvent and in the presence of a Li+ cation, while the hydride transfer is significantly hindered by the presence of cations (Li+ and K+). The cation seems to strongly stabilize the negatively charged formate moiety, hindering complete hydride transfer and resulting in a high barrier for this step. This study is a first step towards addressing the exact role of cations in formic acid dehydrogenation reactions.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...