ILs through the looking glass: electrostatics and structure probed using charge-inverted ionic liquid pairs†
Abstract
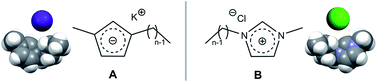
Ionic liquids combining potassium cations with 1-alkyl-3-methylcyclopentadienyl anions, K[CnC1Cp] (n = 4, 6) have been synthesized. Differential scanning calorimetry measurements have shown that K[C4C1Cp] and K[C6C1Cp] melt without decomposition at around 90 °C. These two ionic liquids are the charge-inverted counterparts of [C4C1Im]Cl and [C6C1Im]Cl, two common ionic liquids. The concept of charge-inverted ionic pairs is used to explore the nature of the interactions and structure in different ionic compounds, from simple alkali halide salts to ionic liquids based on complex molecular ions. Different sets of experimental data, empirical correlations and molecular dynamics simulations are used to that effect.
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...