Optical properties and photochemical production of hydroxyl radical and singlet oxygen after ozonation of dissolved organic matter†
Abstract
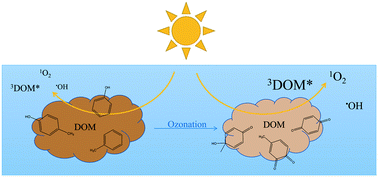
This study focuses on the effects of ozonation on the optical and photochemical properties of dissolved organic matter (DOM). Upon ozonation, a decrease in light absorption properties of DOM was observed concomitantly with a large increase in singlet oxygen (1O2) and hydroxyl radical (˙OH) quantum yields (Φ1O2 and Φ˙OH, respectively). The decrease in absorbance was linked to the reaction of DOM chromophores with ozone and ˙OH, formed as a secondary oxidant, while the increase in Φ1O2 and Φ˙OH are linked to the formation of quinone-like moieties from the reaction of ozone with phenolic DOM moieties. Investigations using benzoic acid as a ˙OH probe and methanol as a ˙OH scavenger indicated that not only is ˙OH formed, but that other hydroxylating species (˙OH-like) are also produced upon DOM photo-irradiation.


 Please wait while we load your content...
Please wait while we load your content...
