Aerobic oxidation of arsenite to arsenate by Cu(ii)–chitosan/O2 in Fenton-like reaction, a XANES investigation†
Abstract
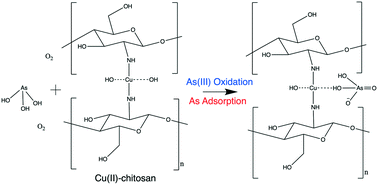
Oxidation of inorganic molecules (e.g., arsenite (As(III)) to arsenate (As(V))) by Cu(II)–chitosan in the presence of dissolved oxygen is examined to elucidate the ability and mechanism of Cu(II)–chitosan and Cu(II)–n-TiO2–chitosan participation in Fenton-like reactions. To form the Cu(II)–chitosan complex, Cu(II) binds with the amine groups of the chitosan backbone resulting in a Cu(II)-complex with cationic behavior. Arsenic is then adsorbed to the copper binding site through a combination of Lewis acid–base coordinate bonding and electrostatics. As K-edge XANES indicate that arsenite is fully oxidized to arsenate when adsorbed to a Cu(II)–chitosan complex in the dark without introduction of a photo- or chemical-oxidant. The oxidation of arsenite by this complex is strongly controlled by the presence of dissolved oxygen as suggested by linear combination fitting where the % As(V) bound decreases from 100% ± 0.0% to 60.2 ± 0.1% upon removal of dissolved oxygen via the freeze pump thaw method. Cu K-edge XANES indicate that Cu(II) acts as a catalyst rather than a reactant, as it remains present as Cu(II) after As oxidation in each system condition examined. For Cu(II)–n-TiO2–chitosan, the amount of As(III) oxidized to As(V) is strongly controlled by the loading of Cu(II), with a higher loading of Cu(II) leading to more As(III) oxidized and bound on the surface of the adsorbent as As(V). As(III) removal by both Cu(II)–chitosan and Cu(II)–nTiO2–chitosan is significantly improved due to the Fenton-like oxidation of As(III) to As(V). For Cu(II)–n-TiO2–chitosan, higher loadings of Cu(II) relative to n-TiO2 lead to greater improvement of As(III) removal performance under oxic vs. anoxic conditions.


 Please wait while we load your content...
Please wait while we load your content...
