Iron-electrocoagulation as a disinfection byproduct control strategy for drinking water treatment†
Abstract
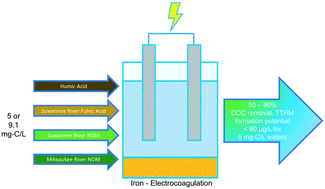
Disinfection byproducts (DBPs) result from reactions between oxidizing disinfectants, e.g., free chlorine, and DBP precursors such as natural organic matter (NOM). Electrocoagulation (EC) has potential as an effective DBP precursor removal strategy for decentralized water treatment and small drinking water utilities as it features similar removal trends compared to conventional coagulation, without alkalinity consumption, while also generating coagulant in situ by anodic dissolution of iron electrodes. However, studies assessing DBP formation in drinking water following EC treatment are lacking. This research evaluated EC as a DBP precursor removal strategy by quantifying reductions in total trihalomethane (TTHM) formation potential as a function of electrolysis time (2.5–17.5 min), pH (6–9.1), and the type of NOM treated (5–9.1 mg-C per L spiked as humic acid, fulvic acid, or NOM, or initially present in the Milwaukee River). Using doses of approximately 35 mg-Fe per L, EC performed similarly to conventional coagulation when the water matrices were at the same pH. As pH decreased, shorter EC electrolysis times were needed to decrease TTHM formation potential to meet the U.S. Environmental Protection Agency maximum contaminant level for TTHMs. EC decreased the mean specific TTHM formation potential (μg-TTHM per mg-C) of every NOM source, thereby demonstrating that the residual NOM following EC treatment formed less DBPs per unit carbon compared to the influent water.


 Please wait while we load your content...
Please wait while we load your content...