Adsorption of organic micropollutants to biosolids-derived biochar: estimation of thermodynamic parameters†
Abstract
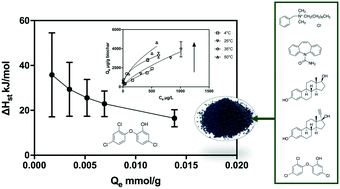
This research quantified thermodynamic parameters to better understand the use of wastewater biosolids-derived biochar as an adsorbent to remove micropollutants. The objective of this research was to quantify adsorption capacity; isosteric heat; and change of enthalpy, entropy, and free energy characterizing adsorption reactions between biochar and micropollutants. Adsorption isotherms were developed using a range of temperatures for the micropollutants benzyldimethyldecylammonium chloride (BAC-C10), carbamazepine (CBZ), 17β-estradiol (E2), 17α-ethynylestradiol (EE2), and triclosan (TCS). The thermodynamic parameters derived from the isotherm data were used to assist in characterizing binding affinity, spontaneity, and mechanisms of adsorption. More polar compounds such as BAC-C10 and CBZ exhibited linear adsorption, indicating weak interactions with more polar amorphous moieties on the biochar surface. For the micropollutants that were present predominantly in the neutral form at pH 7 (CBZ, E2, EE2, and TCS), increasing hydrophobicity increased the extent of adsorption. The enthalpy change of adsorption and the positive correlation between hydrophobicity and change of entropy (R2 = 0.8) both suggest that hydrophobic interaction was the dominant adsorption mechanism for neutral compounds. Increases in adsorption with increasing temperature, together with the estimated thermodynamic parameters, indicated that the reactions were endothermic, meaning that higher temperatures should offer improved removal via adsorption. The negative free energy changes observed suggested that adsorption was spontaneous and that adsorption rates outcompete desorption rates. Under multi-solute conditions, the adsorption capacities for all compounds were suppressed to varying extents; however, the competitive multi-solute adsorption reactions were still spontaneous and endothermic.


 Please wait while we load your content...
Please wait while we load your content...