A framework to analyze sulfate versus chloride selectivity in nanofiltration†
Abstract
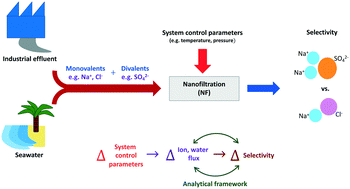
The preferential removal of sodium sulfate over sodium chloride (fractionation) by nanofiltration (NF) is studied. A fractionation metric, M, is defined, and a simple set of equations are derived to describe its variation due to operating parameters, such as temperature and pressure. The use of these equations to explain known behavior of NF systems and suggest improvements in system or membrane design is demonstrated. Furthermore, the derived framework is applicable to all membranes used in pressure-driven technologies, without detailed characterization of specific membrane properties, such as structural parameters (pore radius, active layer thickness, tortuosity, porosity) or charge-based parameters. Given that the principal applications of NF involve the selective removal of multivalent ions over monovalent ions, the introduced metric provides a direct measure of its efficacy in such applications, and is shown to be more accurate in some cases than comparing salt rejection ratios, as usually done. Finally, the concept of ‘breakthrough’, commonly observed as the point at which rejection ratio begins to decrease with increase in pressure, is mathematically described, and its implication on selectivity discussed.


 Please wait while we load your content...
Please wait while we load your content...