Removal of pharmaceuticals by a potassium ferrate(vi) material: from practical implementation to reactivity prediction†
Abstract
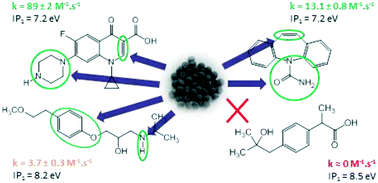
This study investigated the degradation of metoprolol (MET), carbamazepine (CBZ), ciprofloxacin (CIP) and hydroxy-ibuprofen (OH-IBU) in aqueous solution by a ferrate(VI) material obtained by dry synthesis according to patent WO2008065279. Ferrate(VI) had the highest reactivity with CIP, with a second-order rate constant of 89 ± 2 M−1 s−1 at pH 10.3 ± 0.3. The rate constants of ferrate(VI) with MET and CBZ under the same conditions were 3.7 ± 0.3 M−1 s−1 and 13.1 ± 0.8 M−1 s−1, respectively, while no reaction took place with OH-IBU. We also evaluated the removal efficiencies of nine selected pharmaceuticals, including MET, CBZ, CIP and OH-IBU, detected in a real hospital wastewater (HWW) with concentrations ranging from 73 ± 4 ng L−1 to 159 ± 8 μg L−1 by applying Fe(VI) technology. The abatement of the targeted pharmaceuticals depends on their structures. Because Fe(VI) captures electrons during the oxidation process, we proposed to correlate the reactivity of Fe(VI) with the first ionization potential (IP) of the pharmaceuticals. The first IP values of MET, CBZ, CIP, OH-IBU and diclofenac (DFC) were determined by gas-phase UV photoelectron spectroscopy (UV-PES). The UV-PES data were also interpreted using density functional theory (DFT) calculations.


 Please wait while we load your content...
Please wait while we load your content...