Beneficial synergy of adsorption–intercalation–conversion mechanisms in Nb2O5@nitrogen-doped carbon frameworks for promoted removal of metal ions via hybrid capacitive deionization†
Abstract
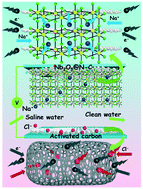
Capacitive deionization (CDI) is an emerging water purification technology, but the ion adsorption capacity of traditional carbon-based CDI electrodes is still unsatisfactory. Herein, a novel faradaic electrode by anchoring Nb2O5 nanoparticles on nitrogen-doped carbon frameworks as anodes and activated carbon (AC) as cathodes in a hybrid capacitive deionization (HCDI) system was originally developed to capture Na+ ions via adsorption–intercalation–conversion mechanisms. The synergistic effects of the nanostructure and carbon coating were beneficial to enhancing electrical conductivity and offering fast Na+ ion diffusion pathways. Impressively, the HCDI system demonstrated an excellent ion adsorption capacity of 35.4 mg g−1 in a 500 mg L−1 NaCl solution at 1.2 V as well as stable regeneration ability. In situ Raman and ex situ XPS measurements unraveled that the mechanism of ion removal from water was the reversible redox reaction of Nb2O5. The new overall understanding of the synergistic effects opens opportunities for the design of HCDI systems for efficient removal of metal ions from saline water.
- This article is part of the themed collection: Nanomaterial applications in water


 Please wait while we load your content...
Please wait while we load your content...