The fabrication of 3D hierarchical flower-like δ-MnO2@COF nanocomposites for the efficient and ultra-fast removal of UO22+ ions from aqueous solution†
Abstract
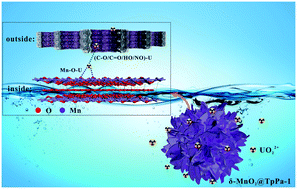
Herein, interpenetrating 3D flower-like δ-MnO2@TpPa-1 composites were suitably constructed though the integration of δ-crystal manganese dioxide (δ-MnO2) nano-flowers with a covalent organic framework (COF, TpPa-1) via adopting a simple ultrasonication process. The physicochemical properties of δ-MnO2@TpPa-1 were characterized via SEM, TEM-EDX, XRD, FT-IR, pHpzc, XPS, and N2 adsorption–desorption studies. The kinetics of UO22+-ion adsorption onto δ-MnO2 and δ-MnO2@TpPa-1 confirmed the existence of a pseudo-second-order model. The results of isothermal experiments showed that the Langmuir model provided a better fit, illustrating a spontaneous, endothermic, and monolayer chemisorption process for UO22+ ions onto δ-MnO2 and δ-MnO2@TpPa-1. The maximum adsorption levels of UO22+ ions onto δ-MnO2 and δ-MnO2@TpPa-1 were 499.41 mg g−1 and 1147.773 mg g−1, respectively, at pH 6.5 and 298 K, owing to electrostatic attraction and inner-sphere surface complexation. Oxygen-containing groups played essential roles in the formation of U–O bonds and covalent Mn–O–U bonds, making δ-MnO2@TpPa-1 an excellent adsorbent for radionuclide elimination from solution.
- This article is part of the themed collection: Environmental Science: Nano Cover Art


 Please wait while we load your content...
Please wait while we load your content...