Enhanced adsorption of Cr(vi) on BiOBr under alkaline conditions: interlayer anion exchange†
Abstract
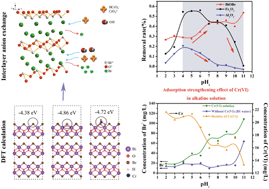
It remains a formidable challenge to remove toxic oxoanions in alkaline conditions. Here, we reveal an enhanced adsorption of Cr(VI) on 3D spherical BiOBr in alkaline conditions. Higher pH values benefit Cr(VI) adsorption on BiOBr, which is a completely different behavior from that displayed by conventional metal-oxide adsorbents. A unique adsorption mechanism of Cr(VI) on BiOBr is proposed for the first time and demonstrated by experimental results and characterization. X-ray photoelectron spectroscopy and Fourier-transform infrared spectroscopy of BiOBr before and after adsorption indicated a Br−–Cr(VI) anion exchange. Variations in the Br− concentrations and pH values of the solutions further verified the interlayer anion exchange between Br− and Cr(VI) ions and the role of OH− in the adsorption process. The presence of OH− promotes the release of Br−, generating cavities for the CrO42− species, thereby significantly improving the adsorption of Cr(VI). More importantly, the anion exchange is theoretically feasible, which was confirmed by density functional theory calculations through the comparison of the adsorption energies. Although the stability and reproducibility of the adsorbent await to be perfected, the present study provides a new perspective on the adsorption of toxic oxoanions in alkaline conditions and uncovers an original adsorption mechanism of BiOXs (X = Cl, Br, I).


 Please wait while we load your content...
Please wait while we load your content...