Experimental and geochemical simulation of nickel carbonate mineral precipitation by carbonate-laden ureolytic fungal culture supernatants†
Abstract
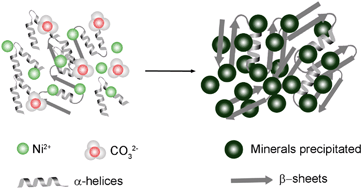
Ureolytic microorganisms have attracted much interest for the bioprecipitation, removal and biorecovery of toxic metals from aqueous solution but little is known about the distribution of cationic/anionic components or quantification of metal species in a fungal system, or the contribution of extracellular protein structure and adsorption during precipitation. In this research, carbonate-rich fungal growth supernatants were applied for bioprecipitation of Ni-containing minerals. The solubility and stability of relevant nickel species was calculated and simulated using Geochemists' Workbench (GWB) and the structure and adsorption of extracellular protein were determined using attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR). The results showed that hydrated nickel carbonate and trace nickel phosphate minerals, with a proportion being of nanoscale dimensions, were precipitated after mixing fungal growth supernatant and NiCl2. The solubility diagrams calculated by GWB showed that under the same condition nickel phosphates were the only precipitate in the simulated system. X-ray photoelectron spectroscopy (XPS) results showed the existence of protein N in the minerals and a protein–nanoparticle bond in C 1s spectra, which showed that extracellular protein might be an important factor in the difference between experimental results and the geochemical simulation. ATR-FTIR showed that during the biomineralization process, the conformation of extracellular proteins changed and β structures increased at the expense of highly ordered α-helixes and other disordered structures, which might provide more nucleation sites to promote the crystallization of nickel carbonate especially under saturated conditions of CO32−. These results demonstrate that the combination of geochemical modelling and experimental analysis provide more complete scientific data to the explain differences between experimental results and theoretical outcomes. This can therefore enable reasonable predictions as to the effect of metal-containing nanoparticles on protein structure, and vice versa, during the process of metal bioprecipitation mediated by a carbonate-rich fungal growth supernatant system.


 Please wait while we load your content...
Please wait while we load your content...