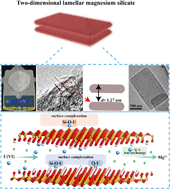
Two-dimensional lamellar magnesium silicate with large spacing as an excellent adsorbent for uranium immobilization†
Abstract
The remediation of poisonous radioactive pollutants in water systems for further treatment and disposal is essential. In this work, two-dimensional (2D) lamellar magnesium silicate nanosheets were built via the in situ conversion of a zeolite precursor, RUB-15. The unique rough surface of magnesium silicate consisted of an infinite number of extremely small nanosheets with a large spacing of 1.27 nm. The magnesium silicate was applied in the immobilization of U(VI) ions in various kinds of wastewater, and possessed high adsorption capacity (the saturated adsorption capacity was 473.83 mg g−1 at 298.15 K), wide pH suitability, high stability at high ionic strength and superior selectivity. A series of batch experiments and characterization indicated that the ion-exchange and surface complexation mechanisms were responsible for the superior adsorption performance. The 2D structure and large spacing ensure that the active sites are well exposed and mass diffusion is fluent, thus promoting the adsorption a lot. Through this low-cost and facile synthesis route, hundreds of grams of magnesium silicate were generated in one batch, giving it a highly promising future for use in the remediation of radioactive U(VI) in the management of aquatic environments.


 Please wait while we load your content...
Please wait while we load your content...