Wrinkle-induced high sorption makes few-layered black phosphorus a superior adsorbent for ionic organic compounds†
Abstract
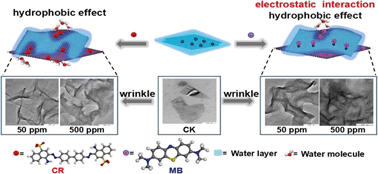
There is a challenge in effectively removing ionic organic compounds from aqueous solution. Inspired by the high drug loading capacity of black phosphorus (BP), here we show that the few-layered BP has high adsorption capacities for both cationic methylene blue (1232 ± 283 mg g−1) and anionic congo red (230 ± 9 mg g−1); these values are among the highest capabilities reported. The maximum theoretical specific surface area of single layered BP is calculated to be 2400 m2 g−1 for the first time. Both electrostatic and hydrophobic interactions are responsible for methylene blue sorption on BP, whereas hydrophobic interactions are the main force for congo red sorption. A sorption kinetic study indicates that the intraparticle diffusion mechanism was involved in both dyes sorption. Most importantly, the wrinkle-induced sorption mechanism was demonstrated by TEM and AFM analyses, and was verified via DFT calculations to account for the high adsorption capacities. These findings demonstrate the promising use of BP as an innovative adsorbent for IOCs in environmental management.


 Please wait while we load your content...
Please wait while we load your content...
