Fractal aggregation and disaggregation of newly formed iron(iii) (hydr)oxide nanoparticles in the presence of natural organic matter and arsenic†
Abstract
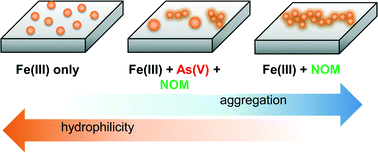
Water chemistry affects the nucleation kinetics, precipitate morphology, and quantity of iron(III) (hydr)oxide nanoparticles, directly impacting the reactive surface area of geomedia and fate of associated waterborne contaminants. In this study, we utilized in situ grazing-incidence small angle X-ray scattering (GISAXS) and complementary ex situ techniques to investigate heterogeneous iron(III) (hydr)oxide nucleation on quartz in the presence of natural organic matter (NOM) and arsenate. Results indicate unique fractal aggregation behavior in the systems containing NOM and precipitating iron(III) (hydr)oxide nanoparticles. Furthermore, the coexistence of arsenic and NOM lead to the formation of two distinct particle size ranges: larger particles dominated by arsenic effects, and smaller particles dominated by NOM effects. These new findings provide important implications for understanding the nucleation, growth, and aggregation of iron(III) (hydr)oxides in aqueous systems where NOM is present, such as natural surface waters and water and wastewater treatment plants. This study also offers new insight into how NOM-associated iron(III) (hydr)oxides can interact with aqueous contaminants such as arsenate.

 Please wait while we load your content...
Please wait while we load your content...