Products and mechanism of thermal decomposition of chlorpyrifos under inert and oxidative conditions†
Abstract
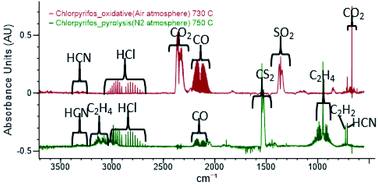
Chlorpyrifos (CPF) is a widely used pesticide; however, limited experimental work has been completed on its thermal decomposition. CPF is known to decompose into 3,5,6-trichloro-2-pyridinol (TCpyol) together with ethylene and HOPOS. Under oxidative conditions TCpyol can decompose into the dioxin-like 2,3,7,8-tetrachloro-[1,4]-dioxinodipyridine (TCDDPy). With CPF on the cusp of being banned in several jurisdictions worldwide, the question might arise as to how to safely eliminate large stockpiles of this pesticide. Thermal methods such as incineration or thermal desorption of pesticide-contaminated soils are often employed. To assess the safety of thermal methods, information about the toxicants arising from thermal treatment is essential. The present flow reactor study reports the products detected under inert and oxidative conditions from the decomposition of CPF representative of thermal treatments and of wildfires in CPF-contaminated vegetation. Ethylene and TCpyol are the initial products formed at temperatures between 550 and 650 °C, although the detection of HOPOS as a reaction product has proven to be elusive. During pyrolysis of CPF in an inert gas, the dominant sulfur-containing product detected from CPF is carbon disulfide. Quantum chemical analysis reveals that ethylene and HOPOS undergo a facile reaction to form thiirane (c-C2H4S) which subsequently undergoes ring opening reactions to form precursors of CS2. At elevated temperatures (>650 °C), TCpyol undergoes both decarbonylation and dehydroxylation reactions together with decomposition of its primary product, TCpyol. A substantial number of toxicants is observed, including HCN and several nitriles, including cyanogen. No CS2 is observed under oxidative conditions – sulfur dioxide is the fate of S in oxidation of CPF, and quantum chemical studies show that SO2 formation is initiated by the reaction between HOPOS and O2. The range of toxicants produced in thermal decomposition of CPF is summarised.


 Please wait while we load your content...
Please wait while we load your content...