Rapid and selective lithium recovery from desalination brine using an electrochemical system†
Abstract
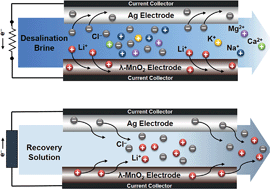
Due to the steep increase in the use of mobile electronics and electric vehicles, there has been a dramatic rise in the global lithium consumption. Although seawater is considered as an ideal future source of lithium, technological advances are necessary to ensure the economic feasibility of lithium recovery from seawater because the concentration and portion of Li+ are extremely low in seawater. Especially, battery-based electrochemical systems for lithium recovery have been considered as promising lithium recovery methods, though they have not been considered for seawater applications due to the extremely low concentration of Li+. In this study, we demonstrate that an electrochemical system based on a battery electrode material (λ-MnO2) can be used for efficient lithium recovery from desalination brine (2–3 times concentrated seawater). Our approach was able to capture Li+ within a substantially short period of time compared to conventional processes at a rate that was at least 3 times faster than that of adsorption processes, and our approach did not require acid or toxic chemicals unlike the other recovery technologies. Moreover, by consecutive operation of the system, a lithium recovery solution containing 190 mM of Li+ was obtained with only a small consumption of energy (3.07 Wh gLi−1), and the purity of Li+ was increased to 99.0%.


 Please wait while we load your content...
Please wait while we load your content...