Reduction of PCE and TCE by magnetite revisited†
Abstract
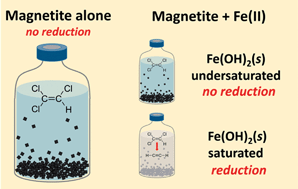
Here we revisit whether the common mixed-valent Fe mineral, magnetite, is a viable reductant for the abiotic natural attenuation of perchloroethylene (PCE) and trichloroethylene (TCE) in anoxic groundwater plumes. We measured PCE and TCE reduction by stoichiometric magnetite as a function of pH and Fe(II) concentration. In the absence of added Fe(II), stoichiometric magnetite does not reduce PCE and TCE over a three month period under anoxic conditions. When Fe(II) is added to magnetite suspensions, PCE and TCE are reduced under Fe(II) and pH conditions that appear to be controlled by the solubility of ferrous hydroxide, Fe(OH)2(s). Reduction rates are slow with only 1 to 30% carbon products (primarily acetylene) accumulating over several months. We conducted a similar set of experiments with Fe(OH)2(s) alone and found that, compared to in the presence of magnetite, Fe(OH)2(s) reduces PCE and TCE only at Fe(II) concentrations that are too high (≥13 mM, 726 mg L−1) to be representative of natural aquifer conditions. Our results suggest that magnetite present in aquifer sediments alone is unlikely to reduce PCE and TCE sufficiently fast to contribute to natural attenuation of PCE and TCE. The lack of compelling evidence for PCE and TCE reduction by magnetite raises important questions regarding the current application of using magnetic susceptibility as a potential indicator for abiotic natural attenuation. Dynamic conditions and high Fe(II) concentrations that favor active precipitation of minerals, such as Fe(OH)2(s) in the presence of magnetite (or other Fe minerals), however, may lead to PCE and TCE reduction that could help attenuate PCE and TCE plumes.


 Please wait while we load your content...
Please wait while we load your content...
