Density functional theory characterization of the structures of H3AsO3 and H3AsO4 adsorption complexes on ferrihydrite†
Abstract
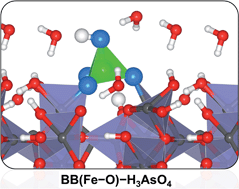
Reactions occurring at ferric oxyhydroxide surfaces play an important role in controlling arsenic bioavailability and mobility in natural aqueous systems. However, the mechanism by which arsenite and arsenate complex with ferrihydrite (Fh) surfaces is not fully understood and although there is clear evidence for inner sphere complexation, the nature of the surface complexes is uncertain. In this work, we have used periodic density functional theory calculations to predict the relative energies, geometries and properties of arsenous acid (H3AsO3) and arsenic acid (H3AsO4), the most prevalent form of As(III) and As(V), respectively, adsorbed on Fh(110) surface at intermediate and high pH conditions. Bidentate binuclear (BB(Fe–O)) corner-sharing complexes are shown to be energetically favoured over monodentate mononuclear complexes (MM(Fe–O)) for both arsenic species. The inclusion of solvation effects by introducing water molecules explicitly near the adsorbing H3AsO3 and H3AsO4 species was found to increase their stability on the Fh surface. The adsorption process is shown to be characterized by hybridization between the interacting surface Fe-d states and the O and As p-states of the adsorbates. Vibrational frequency assignments of the As–O and O–H stretching modes of the adsorbed arsenic species are also presented.


 Please wait while we load your content...
Please wait while we load your content...