Plutonium partitioning in water–granite and water–α-FeOOH systems: from a viewpoint of a three-phase system
Abstract
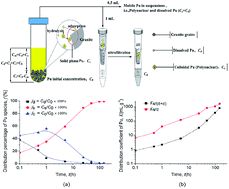
Traditional sorption experiments commonly treat the colloidal species of low-solubility contaminants as immobile species when separated by centrifugation or ultrafiltration. This study shows that, from a viewpoint of a three-phase system, the mobile Pu species, especially the colloidal species, play an important role in Pu partitioning in water–granite and water–α-FeOOH systems. A new distribution coefficient term Ks/(d+c) was defined to take the mobile colloidal species into consideration, and it differs to the traditional distribution coefficient Ks/d by orders of magnitude in the water–granite and water–α-FeOOH systems. This term, Ks/(d+c), can quantitatively describe Pu partitioning in the suspension, in particular the fraction of mobile species that dominate Pu migration in the environment. The effects of ionic strength (I) and pH on the Pu partitioning in water–granite and water–α-FeOOH systems are well interpreted with respect to the zeta potential change of granite grains, α-FeOOH colloid particles and polymeric Pu. It is concluded that the presence of the α-FeOOH colloid with a low concentration (<10 mg L−1) is favorable for the stability of colloidal Pu and leads to large proportion of mobile Pu, especially colloid-associated Pu, which will migrate much faster than dissolved Pu in groundwater.


 Please wait while we load your content...
Please wait while we load your content...