Adsorption of tetracycline to nano-NiO: the effect of co-existing Cu(ii) ions and environmental implications†
Abstract
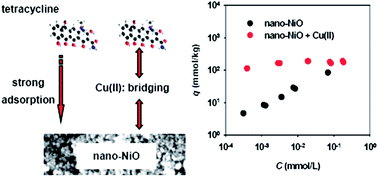
Nano-sized nickel oxide (nano-NiO) is a new nanomaterial that has shown great promise in many areas of application. Understanding its environmental fate and effects is critical for minimizing the potential environmental implications of this new material due to incidental and accidental releases in the future. In this study, we observed strong adsorption of tetracycline to nano-NiO and found that the adsorption affinity can be further enhanced by Cu(II) ions – the observed distribution coefficient (Kd) values are 103.1 to 104.2 L kg−1 in the absence of Cu(II) and 103.0 to 105.5 L kg−1 in the presence of Cu(II); such adsorption affinities are even comparable to those of tetracycline to carbonaceous materials. The strong adsorptive affinities of nano-NiO for tetracycline are likely attributable to several mechanisms, including surface complexation, cation exchange, and electrostatic attraction. As a strong complexing agent, Cu(II) can significantly enhance adsorption of tetracycline by serving as a bridging agent between tetracycline and nano-NiO. The findings of this study have important implications for the risk assessment of engineered nanomaterials – in aquatic environments nano-NiO (and likely other metal oxide nanomaterials) can strongly adsorb tetracycline antibiotics, resulting in the changes of environmental risks of the metal oxide nanomaterials and/or bioavailability of the adsorbed contaminants.

 Please wait while we load your content...
Please wait while we load your content...