Magnetically recoverable fluorescence chemosensor for the adsorption and selective detection of Hg2+ in water†
Abstract
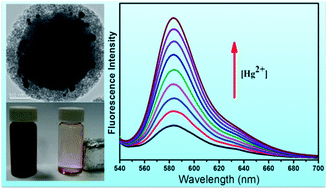
In order to conveniently and effectively detect the heavy metal ion Hg2+ existing in water, a magnetic fluorescence chemosensor has been strategically prepared by immobilizing a Rhodamine B derivative RhB–tris(2-aminoethyl)amine on Fe3O4@SiO2–Au@PSiO2 composites via gold particles. The adsorption and detection for Hg2+ ions were investigated with fluorophotometry. This chemosensor shows high sensitivity and high selectivity for Hg2+ over other metal cations owing to the ring opening of the rhodamine fluorophore selectively induced by Hg2+. In addition, the presence of Fe3O4 in the sensor also facilitates the magnetic separation of the Fe3O4@SiO2–Au–Hg2+@PSiO2 from the solution.

 Please wait while we load your content...
Please wait while we load your content...