The adsorption behavior of U(vi) on granite
Abstract
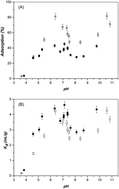
The effects of pH, counter ions and temperature on the adsorption of U(VI) on Beishan granite (BsG) were investigated in the presence and absence of fulvic acid (FA) and humic acid (HA). The adsorption edge of U(VI) on BsG suggested that U(VI) adsorption was mainly controlled by ion exchange and outer-sphere complexation at low pH, whereas inner-sphere complex was the dominant adsorption species in the pH range of 4.0–9.0. Above pH 9.0, Na2U2O7 might play an important role in the rise of U(VI) adsorption again. Counter ions such as Cl−, SO42− and PO43− can provoke U(VI) adsorption on BsG to some extent, which was directly correlated to the complexing ability of U(VI)–ligand. More noticeably, the large enhancement of U(VI) adsorption in the presence of phosphate can be attributed to the ternary complex formation (BsG–PO4–UO2), precipitation ((UO2)3(PO4)2(s)) and secondary phase (Na-autunite). Both FA and HA can slightly increase U(VI) adsorption at low pH, whereas they strongly inhibited U(VI) adsorption at high pH range. Artificial synthesized granite (AsG) prepared in the laboratory is impossible to use as an analogue of natural granite because of the large difference in the adsorption and surface properties.

 Please wait while we load your content...
Please wait while we load your content...