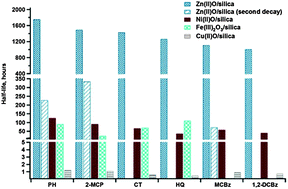
Previous studies indicated that Environmentally Persistent Free Radicals (EPFRs) are formed in the post-flame, cool zone of combustion. They result from the chemisorption of gas-phase products of incomplete combustion (particularly hydroxyl- and chlorine-substituted aromatics) on Cu(II)O, Fe(III)2O3, and Ni(II)O domains of particulate matter (fly ash or soot particles). This study reports our detailed laboratory investigation on the lifetime of EPFRs on Zn(II)O/silica surface. Similarly, as in the case of other transition metals, chemisorption of the adsorbate on the Zn(II)O surface and subsequent transfer of electron from the adsorbate to the metal forms a surface-bound EPFR and a reduced metal ion center. The EPFRs are stabilized by their interaction with the metal oxide domain surface. The half-lives of EPFRs formed on Zn(II)O domains were the longest observed among the transition metal oxides studied and ranged from 3 to 73 days. These half-lives were an order of magnitude longer than those formed on nickel and iron oxides, and were 2 orders of magnitude longer compared to the EPFRs on copper oxide which have half-lives only on the order of hours. The longest-lived radicals on Zn(II)O correspond to the persistency in ambient air particles of almost a year. The half-life of EPFRs was found to correlate with the standard reduction potential of the associated metal.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...