Towards high-areal-capacity aqueous zinc–manganese batteries: promoting MnO2 dissolution by redox mediators†
Abstract
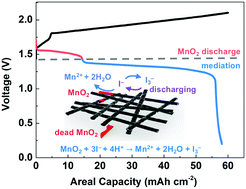
Aqueous manganese (Mn) batteries based on the deposition–dissolution reaction of Mn2+/MnO2(s) have attracted great attention due to their low cost, high voltage, and high safety. However, the incomplete dissolution of MnO2 and exfoliated MnO2 from mechanical cracks of thick MnO2 layers (lost capacity) prevent long-term stable operation at high areal capacity (>2.0 mA h cm−2). Here, we propose a mediator strategy to facilitate MnO2 dissolution and recover ‘lost’ capacity from exfoliated MnO2, which improves the cycling stability at high areal capacity. UV-visible spectroscopy was used to verify the working principle of the mediator strategy. The iodide (I−) mediator chemically reduces solid MnO2 to form Mn2+ and oxidizes to tri-iodide (I3−), which then can get reduced at the electrode returning to I−, completing one mediation cycle. The zinc–manganese (Zn–Mn) battery with the iodide mediator shows improved cycling stability at 2.5 mA h cm−2 (400 vs. 100 cycles, static mode) and 15 mA h cm−2 (225 vs. 60 cycles, flow mode). We further increased the areal capacity and demonstrated 50 mA h cm−2 for more than 50 cycles, which is the highest areal capacity achieved for reported Zn–Mn batteries. This mediator strategy is introduced into aqueous Mn-based batteries for the first time, which could also shed light on other deposition-based batteries to achieve stable operation at high areal capacity.


 Please wait while we load your content...
Please wait while we load your content...