Interfacial water shuffling the intermediates of hydrogen oxidation and evolution reactions in aqueous media†
Abstract
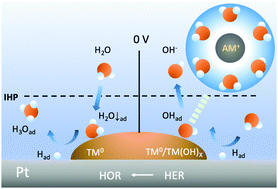
The kinetics of the hydrogen oxidation and evolution reactions (HOR/HER) of platinum in aqueous solutions remains elusive, partly because of the lack of means to explore the surface–electrolyte interface. Herein, we probe this interface by utilizing surface transition metals (TMs), carbon monoxide, alkali metal cations (AM+), and heavy water in combination with in situ X-ray absorption spectroscopy. It was found that the surface TMs in the metallic phase may boost the HOR kinetics of Pt in alkali by hosting the interfacial water with the oxygen-down orientation that removes the adsorbed hydrogen on Pt neighbors. Furthermore, surface TMs in either metallic or hydroxide phases improve the HER kinetics of Pt by hosting the hydroxyl generated from water dissociation so it can be desorbed by the interfacial water coordinated to AM+. The roles of interfacial water in shuffling the HOR/HER intermediates throughout the interface are supported by kinetic isotope effects.


 Please wait while we load your content...
Please wait while we load your content...
