High-efficiency electrochemical hydrodeoxygenation of bio-phenols to hydrocarbon fuels by a superacid-noble metal particle dual-catalyst system†
Abstract
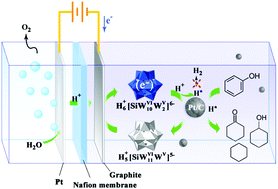
Electrocatalytic hydrogenation (ECH) provides a “green” route to upgrade oxygenated bio-oil under mild conditions, but is still challenged with the issues of low working current density (<60 mA cm−2) and low faradaic efficiency (usually 20–60%) that seriously hinder its practical applications. Herein, we present a dual-catalyst electrochemical route that achieves extremely high faradaic efficiency (>99% for many chemicals) and high working current density (up to 800 mA cm−2) in the hydrogenation of model bio-oil compounds. More importantly, efficient deoxygenation to alkanes, often thought to be very difficult in conventional ECH, was achieved in the aqueous electrolysis. The dual-catalyst system consists of a suspended noble-metal catalyst and soluble polyoxometalate (POM). The theoretical calculations indicate that the POM functions as a superacid, changing the common hydrogenation route to a carbocation mechanism and resulting in effective electrolytic deoxygenation of oxygenates. Because no current flows through the catalyst, even a non-conductive catalyst can be used, which provides a great opportunity for extension to general applications.


 Please wait while we load your content...
Please wait while we load your content...