Singlet oxygen from cation driven superoxide disproportionation and consequences for aprotic metal–O2 batteries†
Abstract
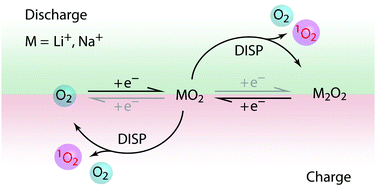
Aprotic alkali metal–oxygen batteries require reversible formation of metal superoxide or peroxide on cycling. Severe parasitic reactions cause poor rechargeability, efficiency, and cycle life and have been shown to be caused by singlet oxygen (1O2) that forms at all stages of cycling. However, its formation mechanism remains unclear. We show that disproportionation of superoxide, the product or intermediate on discharge and charge, to peroxide and oxygen is responsible for 1O2 formation. While the overall reaction is driven by the stability of peroxide and thus favored by stronger Lewis acidic cations such as Li+, the 1O2 fraction is enhanced by weak Lewis acids such as organic cations. Concurrently, the metal peroxide yield drops with increasing 1O2. The results explain a major parasitic pathway during cell cycling and the growing severity in K–, Na–, and Li–O2 cells based on the growing propensity for disproportionation. High capacities and rates with peroxides are now realized to require solution processes, which form peroxide or release O2via disproportionation. The results therefore establish the central dilemma that disproportionation is required for high capacity but also responsible for irreversible reactions. Highly reversible cell operation requires hence finding reaction routes that avoid disproportionation.


 Please wait while we load your content...
Please wait while we load your content...