A GaN:Sn nanoarchitecture integrated on a silicon platform for converting CO2 to HCOOH by photoelectrocatalysis†
Abstract
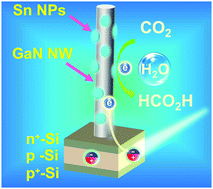
Photoelectrochemical conversion of CO2 with H2O into high-energy fuels and value-added chemicals such as HCOOH provides an appealing strategy for storing solar energy and closing the anthropogenic carbon cycle. However, rational design of catalytic architectures enabling high turnover frequencies (TOFs) for large-scale application has remained a grand challenge. Herein, we report a unique GaN:Sn nanoarchitecture integrated on planar silicon for aqueous photoelectrochemical reduction of CO2 to formic acid. Our density functional theory calculations reveal that the interface of GaN nanowires (NWs) and Sn nanoparticles (NPs), owing to their strong interaction, enables spontaneous CO2 activation, presenting an energetically favorable reaction path for selective HCOOH evolution. Together with the enhanced solar light harvesting, efficient charge carrier extraction, and high catalyst-utilization efficiency, a benchmark TOF of 107 min−1, the highest value ever reported for solar-driven conversion of CO2 to formic acid, is achieved at a high current density of 17.5 mA cm−2, high faradaic efficiency of 76.9%, and low potential of −0.53 V versus reversible hydrogen electrode under one-sun illumination.


 Please wait while we load your content...
Please wait while we load your content...