Asymmetric allyl-activation of organosulfides for high-energy reversible redox flow batteries†
Abstract
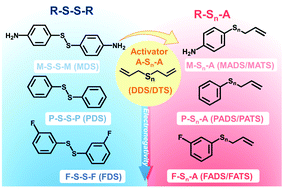
Organosulfides (R–Sn–R) exhibit a high theoretical capacity and low cost, but suffer from sluggish kinetics and poor reversibility. Here, we demonstrate an effective and universal strategy to improve their redox activity by replacing one organic functional group with an allyl (A) to form asymmetric allyl-substituted organosulfides (R–Sn–A). This promotes the cleavage of the A–S bond owing to facile generation of allyl radials, thereby improving the reduction kinetics and capacity. We verified the formation of asymmetric organosulfides by gas-chromatography–mass spectrometry and show that the allyl-activation strategy is universal to a series of functional groups. We successfully increased the discharge potential (up to 280 mV) and discharge capacity (up to 200%) of the organosulfide compared to its symmetric counterpart. A highly-concentrated liquid electrolyte (5 M) with a volumetric capacity of 224 A h L−1 was realized, demonstrating a substantial improvement in volumetric capacity compared to other reported liquid electrolytes to date. This strategy revitalizes organosulfides for high-energy and low-cost energy storage applications.


 Please wait while we load your content...
Please wait while we load your content...