Critical design factors for kinetically favorable P-based compounds toward alloying with Na ions for high-power sodium-ion batteries†
Abstract
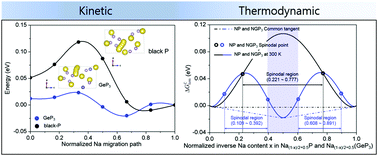
First-principles calculations with experimental validation enable us to radically predict and compare the electron transport, elastic softness, and thermodynamic phase stabilities of black phosphorus (BP) and GeP3 to figure out the crucial factors for designing the most kinetically favorable P-based compounds toward alloying with Na ions. From the viewpoint of electron transport, Ge is able to provide P with electrons due to the difference of electronegativity, causing GeP3 to have a significantly reduced band gap and a lower electron-hopping barrier compared to BP. Because the structure of GeP3 looks more elastically softened than that of BP, its Na+ migration barriers in the sodiated states must be lower than those of the sodiated BP structure as proven by the higher Na+ diffusion coefficients of GeP3. The thermodynamic phase stabilities of BP and GeP3 could be predicted by calculating their mixing enthalpy values and thereby homogeneous bulk free energies were obtained for Na1−x(GeP3) (0.0 ≤ x ≤ 0.5) indicating that no phase separation occurs in the sodiated GeP3 because of an additional stable single-phase at x = 0.25. By contrast, the sodiated P easily gets separated into NaP and Na0.5P with the same sodiation content. The suppressed phase separation in GeP3 makes the Na+ distribution homogeneous as confirmed by TEM-EDS color mapping and thus contributes to increasing Na+ diffusion coefficients because Na+ migration can be primarily interrupted by the interfaces or grain boundaries between the separated phases. Taking into account three enhanced physicochemical properties, the GeP3 electrodes may well present a superior rate capability to the BP electrode, underscoring that electron transport, elastic softness, and thermodynamic phase stabilities must be the most important design factors for realizing kinetically favorable P-based compounds toward alloying with Na+. In detail, even at an ultrahigh current density of 20 A g−1, the capacity of the GeP3 electrode still comes to 0.197 A h g−1. At 1 A g−1, GeP3 could maintain a discharge capacity of 0.499 A h g−1 even after 1000 cycles. This study may provide promising guidelines for the design of alloy-based electrode materials for high power and energy SIBs.


 Please wait while we load your content...
Please wait while we load your content...