Nanostructures of solid electrolyte interphases and their consequences for microsized Sn anodes in sodium ion batteries†
Abstract
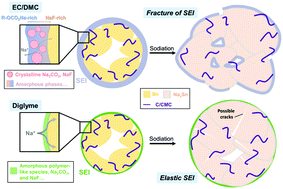
There has been increasing evidence that ether-based electrolytes offer more stable anode performance in sodium ion batteries, even for microsized alloy electrodes which suffer huge volume change upon charge/discharge cycling. It is speculated that ether-based electrolytes may give rise to more robust solid electrolyte interphases (SEIs), but the detailed mechanism remains unknown, due to the structural complexity and the extremely vulnerable nature of SEIs. In this work, we unveil the characteristic SEI structure at Sn electrode surfaces in both carbonate- and ether-based electrolytes. We adopt cryogenic transmission electron microscopy to probe the pristine SEI structure, in combination with X-ray photoelectron spectroscopy and density functional theory calculations. An ultrathin SEI forms in the ether-based electrolyte, with amorphous particles dispersed in the polymer-like matrix. This unique nanostructure exhibits superior mechanical elasticity and renders anomalous stability against the large volume change of alloy electrodes, as evidenced by both electrochemistry measurement and atomic force microscopy. Our work unravels the causes for the superiority of ether-based electrolytes in sodium-ion batteries, and we expect the potential of such an optimized SEI to enable the application of high-capacity anodes such as microsized alloys.


 Please wait while we load your content...
Please wait while we load your content...