High-performance all-solid-state Li–Se batteries induced by sulfide electrolytes†
Abstract
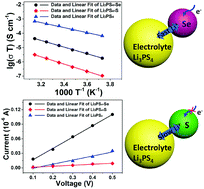
All-solid-state Li–S batteries have attracted significant attention due to their high energy density and high safety. However, the insulating nature of S has limited the electrochemical performance of all-solid-state systems. Alternatively, Se, possessing orders of magnitude higher electronic conductivities, may be a promising cathode candidate but has been completely overlooked in all-solid-state battery systems. Herein, a feasible all-solid-state Li–Se battery is demonstrated using Se–Li3PS4–C as the cathode, Li3PS4 as the electrolyte, and an Li–Sn alloy as the anode. In addition to the high electronic conductivity (1 × 10−3 S cm−1) of Se, a high Li+ conductivity of 1.4 × 10−5 S cm−1 across the Se–Li3PS4 interface can be achieved. The all-solid-state Li–Se cell shows a high reversible capacity of 652 mA h g−1 (96% of theoretical capacity) and exhibits favorable capacity retention upon cycling. This work demonstrates the advantages of a Se cathode in all-solid-state batteries and provides new opportunities for improving the charge transfer of S cathodes in solid-state batteries.


 Please wait while we load your content...
Please wait while we load your content...