Boosting water oxidation on metal-free carbon nanotubes via directional interfacial charge-transfer induced by an adsorbed polyelectrolyte†
Abstract
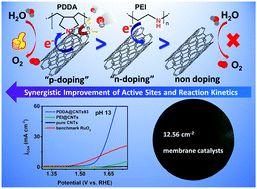
Engineering the surface and electronic structures of nanocarbons is a viable strategy to boost their catalytic performance. Herein, rather than conventional covalent doping, we used the concept of interfacial charge transfer doping of carbon nanotubes (CNTs) to noncovalently adsorb certain polyelectrolytes (i.e. poly(diallyldimethylammonium chloride), PDDA, acceptor) onto pure CNTs (donor) to act as a buffer layer for richening reactants (OH−) via electrostatic interaction and to induce notable interfacial charge redistribution via directional intermolecular charge-transfer, which not only improves the reaction kinetics but also creates a high density of catalytic carbon sites, eventually transforming the inactive CNTs into efficient metal-free water oxidation catalysts. As expected, the resulting PDDA-adsorbed CNT catalysts yielded a remarkably lower overpotential of 370 mV at 10.0 mA cm−2 with a smaller Tafel slope of 76 mV dec−1 with respect to pure CNTs (>520 mV, 166 mV dec−1), even on a par with the benchmark RuO2 (360 mV, 137 mV dec−1) in 0.1 M KOH.


 Please wait while we load your content...
Please wait while we load your content...