Unlocking the potential of graphene for water oxidation using an orbital hybridization strategy†
Abstract
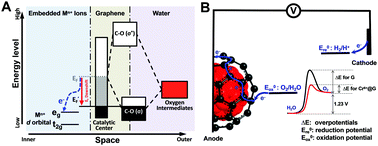
Graphene-based electrocatalytic materials are potential low-cost electrocatalysts for the oxygen evolution reaction (OER). However, substantial overpotentials above thermodynamic requirements limit their efficiency and stability in OER-related energy conversion and storage technologies. Here, we embedded CrN crystals into graphene and in situ electrochemically oxidized them to construct graphene materials with encapsulated Cr6+ ions (Cr6+@G). These Cr6+@G materials exhibit the lowest OER overpotential of 197 mV at 10 mA cm−2 and excellent stability over 200 h at a high current density of about 120 mA cm−2 in an alkaline electrolyte. Spectroscopic and computational studies confirm a stable ion coordination environment significantly benefiting the downshift of the graphene Fermi level via hybridization of C p orbitals with d orbitals of Cr6+ ions that enhances the OER activity and stability.


 Please wait while we load your content...
Please wait while we load your content...