The critical role of lithium nitrate in the gas evolution of lithium–sulfur batteries†
Abstract
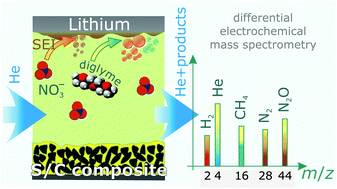
Sulfur–carbon composites are promising next generation cathode materials for high energy density lithium batteries and thus, their discharge and charge properties have been studied with increasing intensity in recent years. While the sulfur-based redox reactions are reasonably well understood, the knowledge of deleterious side reactions in lithium–sulfur batteries is still limited. In particular, the gassing behavior has not yet been investigated, although it is known that lithium metal readily reacts with the commonly used ethereal electrolytes. Herein, we describe, for the first time, gas evolution in operating lithium–sulfur cells with a diglyme-based electrolyte and evaluate the effect of the polysulfide shuttle-suppressing additive LiNO3. The use of the combination of two operando techniques (pressure measurements and online continuous flow differential electrochemical mass spectrometry coupled with infrared spectroscopy) demonstrates that the additive dramatically reduces, but does not completely eliminate gassing. The major increase in pressure occurs during charge, immediately after fresh lithium is deposited, but there are differences in gas generation during cycling depending on the addition of LiNO3. Cells with LiNO3 show evolution of N2 and N2O in addition to CH4 and H2, the latter being the main volatile decomposition products. Collectively, these results provide novel insight into the important function of LiNO3 as a stabilizing additive in lithium–sulfur batteries.

 Please wait while we load your content...
Please wait while we load your content...