Exploring the electrochemical reaction mechanism of carbonate oxidation in Li–air/CO2 battery through tracing missing oxygen†
Abstract
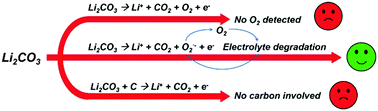
As energy storage systems with great prospects, both Li–air and Li–CO2 batteries possess low energy efficiency and poor cycle performance, which is caused by the accumulation of Li2CO3 during cycling. Therefore, the complete electrochemical decomposition of Li2CO3 during charging will greatly improve the performance of Li–air and Li–CO2 batteries. However, our understanding of the electrochemical decomposition mechanism of Li2CO3 is very limited. In this work, we report that CO2 is released during the electrochemical decomposition of Li2CO3 while O2 is not detected throughout the whole process. Mass spectra and FTIR results show that the electrolyte solvent undergoes degradation during charging. We further demonstrate that this degradation is caused by superoxide radicals, which are generated from Li2CO3.

 Please wait while we load your content...
Please wait while we load your content...