Dramatically enhanced reversibility of Li2O in SnO2-based electrodes: the effect of nanostructure on high initial reversible capacity†
Abstract
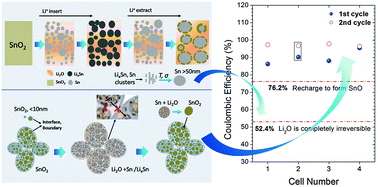
The formation of irreversible Li2O during discharge is believed to be the main cause of large capacity loss and low Coulombic efficiency of oxide negative electrodes for Li batteries. This assumption may have misguided the development of high-capacity SnO2-based anodes in recent years. Here we demonstrated that contrary to this perception, Li2O can indeed be highly reversible in a SnO2 electrode with controlled nanostructure and achieved an initial Coulombic efficiency of ∼95.5%, much higher than that previously believed to be possible (52.4%). In situ spectroscopic and diffraction analyses corroborate highly reversible electrochemical cycling, suggesting that the interfaces and grain boundaries of nano-sized SnO2 may suppress the coarsening of Sn and enable the conversion between Li2O and Sn to amorphous SnO2 when de-lithiated. These results provide important insight into the rational design of high-performance oxide electrodes for Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...