Structural basis for differing electrocatalytic water oxidation by the cubic, layered and spinel forms of lithium cobalt oxides†
Abstract
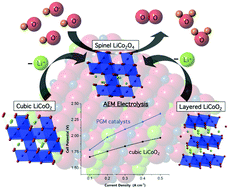
The two polymorphs of lithium cobalt oxide, LiCoO2, present an opportunity to contrast the structural requirements for reversible charge storage (battery function) vs. catalysis of water oxidation/oxygen evolution (OER; 2H2O → O2 + 4H+ + 4e−). Previously, we reported high OER electrocatalytic activity from nanocrystals of the cubic phase vs. poor activity from the layered phase – the archetypal lithium-ion battery cathode. Here we apply transmission electron microscopy, electron diffraction, voltammetry and elemental analysis under OER electrolysis conditions to show that labile Li+ ions partially deintercalate from layered LiCoO2, initiating structural reorganization to the cubic spinel LiCo2O4, in parallel with formation of a more active catalytic phase. Comparison of cubic LiCoO2 (50 nm) to iridium (5 nm) nanoparticles for OER catalysis (commercial benchmark for membrane-based systems) in basic and neutral electrolyte reveals excellent performance in terms of Tafel slope (48 mV dec−1), overpotential (η = ∼420 mV@10 mA cm−2 at pH = 14), faradaic yield (100%) and OER stability (no loss in 14 hours). The inherent OER activity of cubic LiCoO2 and spinel LiCo2O4 is attributed to the presence of [Co4O4]n+ cubane structural units, which provide lower oxidation potential to Co4+ and lower inter-cubane hole mobility. By contrast, the layered phase, which lacks cubane units, exhibits extensive intra-planar hole delocalization which entropically hinders the four electron/hole concerted OER reaction. An essential distinguishing trait of a truly relevant catalyst is efficient continuous operation in a real electrolyzer stack. Initial trials of cubic LiCoO2 in a solid electrolyte alkaline membrane electrolyzer indicate continuous operation for 1000 hours (without failure) at current densities up to 400 mA cm−2 and overpotential lower than proven PGM (platinum group metal) catalysts.

 Please wait while we load your content...
Please wait while we load your content...