Pt–Ru catalyzed hydrogen oxidation in alkaline media: oxophilic effect or electronic effect?†
Abstract
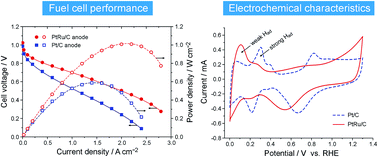
A current challenge to alkaline polymer electrolyte fuel cells (APEFCs) is the unexpectedly sluggish kinetics of the hydrogen oxidation reaction (HOR). A recently proposed resolution is to enhance the oxophilicity of the catalyst, so as to remove the Had intermediate through the reaction with OHad, but this approach is questioned by other researchers. Here we report a clear and convincing test on this problem. By using PtRu/C as the HOR catalyst for the APEFC, the peak power density is boosted to 1.0 W cm−2, in comparison to 0.6 W cm−2 when using Pt/C as the anode catalyst. Such a remarkable improvement, however, can hardly be explained as an oxophilic effect, because, as monitored by CO stripping, reactive hydroxyl species can generate on certain sites of the Pt surface at more negative potentials than on the PtRu surface in KOH solution. Rather, the incorporation of Ru has posed an electronic effect on weakening the Pt–Had interaction, as revealed by the voltammetric behavior and from density-functional calculations, which thus benefits the oxidative desorption of Had, the rate determining step of HOR in alkaline media. These findings further our fundamental understanding of the HOR catalysis, and cast new light on the exploration of better catalysts for APEFCs.

 Please wait while we load your content...
Please wait while we load your content...