Equilibrium voltage and overpotential variation of nonaqueous Li–O2 batteries using the galvanostatic intermittent titration technique†
Abstract
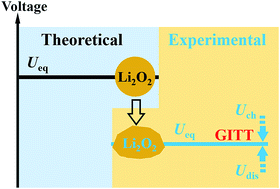
The Li–air (or Li–O2) battery has attracted wide attention, since it has the highest theoretical specific gravimetric energy density. In spite of the rapid progress made on improving its cyclic performance and reducing its voltage polarization, many key issues on thermodynamics and kinetics in nonaqueous Li–O2 batteries are still unresolved. In this study, by using the galvanostatic intermittent titration technique, several novel phenomena have been observed, such as zero voltage gap for the open circuit voltage (OCV) between charging and discharging, asymmetrical polarization behaviours at different current densities and temperatures, a continuous increase of overpotential during charging, and a negative temperature coefficient of the cell's thermodynamic equilibrium voltage. These results could inspire other researchers to comprehensively investigate the complicated reaction mechanisms, thermodynamics, and kinetic properties of the Li–air battery, as well as other advanced batteries.

 Please wait while we load your content...
Please wait while we load your content...