Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass†
Abstract
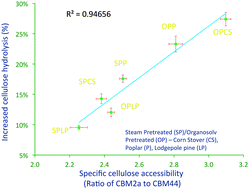
The polysaccharide monooxygenase enzyme AA9 (formerly known as GH61) was shown to interact synergistically with cellulases to enhance the enzymatic hydrolysis of a range of “commercially-relevant” pretreated and “model” cellulosic substrates. Although an exogenous source of reducing power was required when AA9 was added with cellulases to a “pure” cellulosic substrate, it was not required when added to pretreated lignocellulosic substrates. It appears that the non-cellulosic components such as soluble components, lignin, and possibly hemicellulose, can all act as AA9 reducing cofactor. Of the various substrate characteristics that influenced the efficacy of the enzyme mixture, the relative amount of accessible crystalline cellulose, assessed by the specific cellulose binding module (CBM), appeared to be the most critical. Cellulases and AA9 acted synergistically when hydrolysing cellulose I but it did not occur during the hydrolysis of cellulose II and III.

 Please wait while we load your content...
Please wait while we load your content...